
In order to implement the instructions of General Secretary Xi Jinping on strengthening the protection of intellectual property rights, deepen the reforms to streamline the government, delegate power, and improve government services, respond to the demands of examination rules for rapid economic and technological development, and improve the quality and efficiency of patent examination. The China National Intellectual Property Administration has decided to amend the Guidelines for Patent Examination, which are hereby promulgated and shall come into force on January 15, 2021.
Hereby Noticed.
China National Intellectual Property Administration
December 11, 2020
The Decision of the China National Intellectual Property Administration on Amending the Guidelines for Patent Examination
The China National Intellectual Property Administration decided to make the following amendments to the Guidelines for Patent Examination:
Amend Section 3.5 of Chapter 10 of Part 2 of the Guidelines for Patent Examination to read as follows:
3.5 About the submission of the Supplementary Experimental data
3.5.1 Principles of examination
To determine whether the specification is fully disclosed based on the content recorded in the original specification and claims.
The examiner shall examine the experimental data supplemented by the applicant to meet the requirements of Item 3, Article 22 and Item 3, Article 26 of the Patent Law after the application date. The technical effect proved by the supplementary experimental data should be obtained from the disclosure of the patent application by technicians in the same technical field.
3.5.2 The submission of the Supplementary Experimental data for drug patent applications
Examples of examination involving drug patent applications shall be given in accordance with the principles of examination in Section 3.5.1 of this chapter.
【example 1】
The patent claim requests the protection of compound A. The specification records the preparation example of compound A, the effect of reducing blood pressure, and the experimental method for measuring the blood pressure lowering activity without the experimental result data. To prove that the specification is fully disclosed, the applicant supplemented data of Compound A of the blood pressure lowering effect. For those technicians in the same technical field, according to the original application documents, the blood pressure-lowering effect of Compound A has been disclosed, and the technical effect proved by the supplementary experimental data can be obtained from the disclosure of the patent application documents. It should be noted that the supplementary experimental data should also be examined when examining inventiveness.
【Example 2】
The patent claim requests the protection of general formula I compound. The specification records the general formula I and its preparation method, the preparation examples of multiple specific compounds A, B, etc. It also records the anti-tumor effect of general formula I, the experimental method of tumor activity and its experimental result data which is recorded as the IC50 value of the compound of the example on tumor cells falls in the range of 10-100nM. To prove the inventiveness of the claim, the applicant submitted the comparative experimental data, which showed that the IC50 value of compound A was 15nM, while the compound of comparative document 1 was 87nM. For those technicians in the same technical field, according to the original application documents, compound A and its anti-tumor effects has been disclosed, and the technical effect proved by the supplementary experimental data can be obtained from the disclosure of the patent application documents. It should be noted that at this time, the examiner also needs to further analyze whether the technical proposal requested by the claim meets the requirements of inventiveness in conjunction with the supplementary experimental data.
The content of the last paragraph of Section 4.2.3 of Chapter 10 of Part 2 in the Guidelines for Patent Examination, which is “that should be written as performance-limited or use-limited", shall be modified as "that usually need to be written as performance-limited or use-limited". The expression "In some fields, such as alloys, the inherent properties and/or uses of the invention alloys should usually be stated.” Shall be amended to "In some fields, such as alloys, the inherent properties and/or applications of the invention alloys should usually be stated.".
The rest of this section has not been modified.
Amend item (1) of Chapter 10 of Section 5.1 of Part 2 of in the Guidelines for Patent Examinationto read as follows:
(1) If a compound claimed by an patent application has been found that its chemical name, molecular formula (or structural formula) and other structural information are recorded in a comparative document, the technicians in the same technical field will think that the claimed compound has been disclosed and the compound is not novel, unless the applicant can provide evidence to prove that the compound cannot be obtained before the application date.
If the structural information recorded in a comparative document is not sufficient to determine the structural similarities and differences between the claimed compound and the compound disclosed in the comparative document, in combination with other information recorded in the comparative document, including physical and chemical parameters, preparation methods and effect experimental data, the technicians in the same technical field have good reason to presume that the two compounds are substantially the same. That means the claimed compound is not novel, unless the applicant can provide evidence to prove that the structure is indeed different.
The rest of this section has not been modified.
Amend Section 6.1 of Chapter 10 of Part 2 in the Guidelines for Patent Examination to read as follows:
6.1 Inventiveness of the compound
(1) To judge the inventiveness of an invention of a compound, it is necessary to determine the structural difference between the claimed compound and the compound closest to the prior art, and to determine the technical problem actually solved by the invention based on the use and/or effect obtained by the structural modification. On this basis, it could be judged whether the existing technology as a whole provides technical enlightenment for solving the technical problem through its structural transformation.
It should be noted that if the technicians in the same technical field can carry out such structural modification to solve the technical problem and obtain the claimed compound only through logical analysis, reasoning or limited experiment on the basis of the existing technology, it is believed that the existing technology has technical enlightenment.
(2) The use and/or the effect brought about by the structural modification of the compound closest to the prior art by the invention may be to obtain a different use from the known compound, or it may be an improvement in a certain aspect of the effect of the known compound. When judging the inventiveness of a compound, if the change of use and/or the improvement of the effect is unexpected, it is reflected that the claimed compound is non-obvious, and its inventiveness should be recognized.
(3) It should be noted that when judging the inventiveness of a compound invention, if the effect of the claimed technical proposal is caused by a known inevitable trend, the technical proposal is not inventive. For instance, there is an insecticide A-R in existing technology, wherein R is a C1-3 alkyl, and it has been pointed out that the insecticidal effect increases as the number of C atoms of the alkyl increases. If an applied insecticide A-C4H9 has significantly higher insecticidal effect than that of the prior art, the application is not creative since the inevitable trend of improving the insecticidal effect is pointed out at the prior art.
(4) Examples of inventiveness judgment.
【Example 1】
The existing technology:
(Ia)
The application:
(Ib)
The core structures of (Ib) and (Ia) are different, but they have the same purpose. The technicians in the same technical field generally think that compounds with similar structures, which means their compounds have the same basic core part or basic ring, have the same or similar uses. There is no technical enlightenment that obtain (Ib) through modifying the basic ring of (Ia) with the use remains unchanged, so (Ib) is creative.
【Example 2】
The existing technology:H2N-C6H4-SO2NHR1 (Ⅱa)
The application: H2N-C6H4-SO2-NHCONHR1 (Ⅱb)
(IIb) is inserted a -CONH- in the (IIa) NHR1 structural fragment. The uses of these two are completely different. The (IIa) sulfonamide is an antibiotic, while (IIb) sulfonylurea is an antidiabetic drug. The technicians in the same technical field are not motivated to transform R1 in antibiotics into CONHR1 to obtain antidiabetic drugs, so (IIb) is creative.
【Example 3】
The existing technology:H2N-C6H4-SO2NHCONHR1(Ⅲa)
The application: H3C-C6H4-SO2NHCONHR1(Ⅲb)
(Ⅲa) Amino-sulfonylurea and (Ⅲb) methyl-sulfonylurea only differ in the structures of NH2 and CH3, which are both antidiabetic drugs with equivalent effects. Compared to (Ⅲa), (Ⅲb) provides another anti-diabetic drug in this field. Since NH2 and CH3 are classic univalent isosteres, the technicians in the same technical field are motivated to perform the isostere replacement in order to obtain the same or equivalent antidiabetic activity, so (Ⅲb) is not creative.
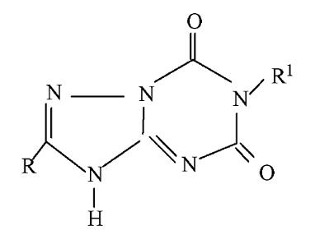
【Example 4】
The existing technology:
(Ⅳa)
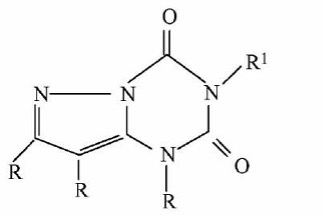
The application:
(Ⅳb)
The only difference between compound (IVb) and compound (IVa) is that the -O- replaces -NH- at the 6-position of the purine. Although -O- and -NH- are classical isosteres that are well known in the field, the cancer cell growth inhibitory activity of (IVb) is about 40 times higher than that of (IVa), and thus (IVb) has achieved unexpected technical effect compared to (IVa). (IVb) is non-obvious, so it is creative.
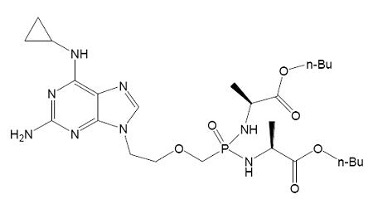
【Example 5】
The existing technology:
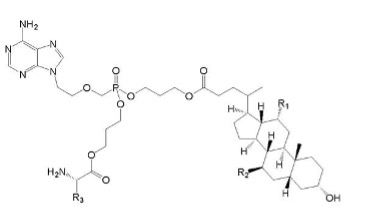
(Ⅴa)
Where R1=OH, R2=H and R3=CH2CH(CH3)2.
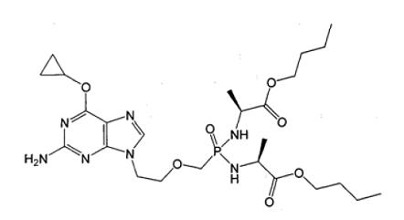
The application:
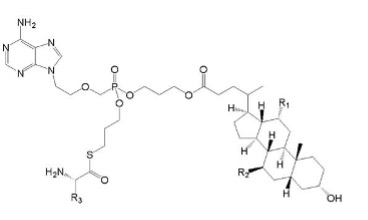
(Ⅴb)
Wherein R1 and R2 are selected from H or OH, R3 is selected from C1-6 alkyl, and includes specific compounds in which R1=OH, R2=H and R3=CHCH3CH2CH3 (Vb1). And the anti-hepatitis B virus activity of (Vb1) is significantly better than (Va).
When the compound of the general formula (Vb) is claimed, the only difference between (Vb) and (Va) is the linking atom between the phosphoryl alkyl and the amino acid residue, in which (Vb) is -S- and (Va) is -O-. Compared to (Va), the compound of general formula (Vb) provides another anti-hepatitis B virus drug in the technical field. Since -S- and -O- are close in nature, in order to obtain other drugs with the same anti-hepatitis B virus activity, the technicians in the same technical field have the motivation to make this substitution and obtain the compound of the general formula (Ⅴb), so (Ⅴb) is not creative.
When the specific compound (Vb1) is claimed, the difference between (Vb1) and (Va) is not only the above-mentioned linking atom, but also the substituent at the R3 position. The anti-hepatitis B virus activity of (Vb1) is significantly better than that of (Va). There is no technical enlightenment to improve the anti-hepatitis B virus activity through the structural modification in the existing technology, so (Vb1) is creative.
"Including the General Microbiology Center of the Chinese Microbial Culture Collection Management Committee (CGMCC) located in Beijing, and China Center for Type Culture Collection (CCTCC) located in Wuhan” in Item4, Section 9.2.2, Chapter 10, Part 2 of the Guidelines for Patent Examination are amended to “Including the General Microbiology Center of the Chinese Microbial Culture Collection Management Committee (CGMCC) located in Beijing, China Center for Type Culture Collection (CCTCC) located in Wuhan, and Guangdong Microbial Culture Preservation Center located in Guangzhou (GDMCC)."
The rest of this section has not been modified.
VI. Amendments to Section 9.3.1.7, Chapter 10, Part 2
Amend Section 9.3.1.7, Chapter 10, Part 2 of the Guidelines for Patent Examination to:
9.3.1.7 Monoclonal antibodies
The claim for a monoclonal antibody can be defined by structural features or by the hybridoma that produces it.
【Examples】
(1) A monoclonal antibody against antigen A, which comprises amino acid sequences VHCDR1, VHCDR2, and VHCDR3 as shown in SEQ ID NO: 1-3, as well as the amino acid sequences VLCDR1, VLCDR2, and VLCDR3 as shown in SEQ ID NO: 4-6 .
(2) The monoclonal antibodies against antigen A are produced by the hybridomas with deposit number CGMCC NO:xxx.
VII. Amendments to Section 9.4.2, Chapter 10, Part 2
(1) Three new paragraphs are added under the title of Inventiveness of Section 9.4.2 of Chapter 10 of Part 2 of the Guidelines for Patent Examination, the content is as follows:
The judge of the inventiveness of an invention in the field of biotechnology also needs the judge of whether the invention has outstanding substantive features and significant progress. In the judging process, it is necessary to determine the distinguishing features between the invention and the closest prior art based on the specific limitations of different protection subjects. And then we can judge whether the existing technology as a whole gives technical enlightenment based on the technical effect that the distinguishing feature can achieve in the invention and the technical problem actually solved by the invention. After that, it can be found whether the invention is obvious compared to the existing technology.
Inventions and creations in the field of biotechnology involve protection topics of different levels, such as biological macromolecules, cells, and individual microorganisms. In the ways of characterizing these protection topics, in addition to common methods such as structure and composition, special methods such as the deposit number of biological materials are also included. Inventive judgments need to consider the structural differences between the invention and the existing technology, the distance between the kinship, and the predictability of technical effect.
Some specific situations in the inventive judgments of different protection topics in this field are shown as follows.
(2) Amend Item 1, Article 9.4.2.1, Chapter 10, Part 2 of the Guidelines for Patent Examination to:
(1) Gene
If a protein encoded by a structural gene has a different amino acid sequence and a different type of or improved performance compared to a known protein, besides, and the existing technology does not provide the technical enlightenment of the above-mentioned performance changes caused by the sequence difference, then the gene invention that encodes the protein is creative.
If the amino acid sequence of a certain protein is known, the invention of the gene encoding the protein is not inventive. If a protein is known but its amino acid sequence is unknown, as long as the technician in this field can easily determine its amino acid sequence when the application is filed, the invention of the gene encoding the protein is not inventive. However, in the above two cases, if the gene has a specific base sequence, and has an effect that technician in this field would not expect compared with other protein from genes encoding with a different base sequence, the invention of genes is creative.
If the structural gene claimed by an invention is a naturally available mutant structural gene of a known structural gene, the claimed structural gene is derived from the species that same as the known structural gene, and they also have the same properties and functions, the invention is not creative.
(3) Add the item 2 of peptide or protein to the Article 9.4.2.1, Chapter 10, Part 2 of the Guidelines for Patent Examination as follows.
2) Peptides or proteins
If the polypeptide or protein claimed by the invention is different from the known polypeptide or protein in amino acid sequence, having different types of or improved properties, and the existing technology does not provide the technical enlightenment of the above-mentioned performance changes caused by the sequence difference, then the invention of the polypeptide or protein is creative
(4) Amend "(2) Recombinant vector" in Section 9.4.2.1 of Chapter 10 of Part 2 of the Guidelines for Patent Examination to "(3) Recombinant vector", and insert a paragraph before the original content, with the content as follows:
If the invention aims at the structural modification of known vectors and/or inserted genes improve the performance of the recombinant vector, and the prior art does not provide technical enlightenment for improving performance using the above-mentioned structural modification, the invention of the recombinant vector is inventive.
(5) Amend "(3) Transformant" in Section 9.4.2.1 of Chapter 10 of Part 2 of the Guidelines for Patent Examination to "(4) Transformant ", and insert a paragraph before the original content, with the content as follows:
If the invention aims at the structural modification of a known host and/or inserted gene improve the performance of the transformant, and the prior art does not provide technical enlightenment for improving performance using the above-mentioned structural modification, the invention of the transformant is inventive.
(6) Amend "(4) Fusion Cells" in Section 9.4.2.1, Chapter 10, Part 2 of the Guidelines for Patent Examination to "(5) Fusion Cells".
(7) Amend "(5) Monoclonal Antibody" in Section 9.4.2.1, Chapter 10, Part 2 of the Guidelines for Patent Examination to "(6) Monoclonal Antibody", and amend the whole content as follows:
If the antigen is known, the monoclonal antibody of this antigen characterized by structural features is obviously different from the known monoclonal antibody in the key sequence that determines the function and use, and the prior art does not provide technical enlightenment for obtaining the monoclonal antibody with the above sequence, and the monoclonal antibody can produce beneficial technical effects, the invention of the monoclonal antibody is creative.
If the antigen is known, and the antigen is clearly immunogenic (for example, if the polyclonal antibody of the antigen is known or the antigen is a macromolecular polypeptide, it can be known that the antigen is obviously immunogenic), then the invention of monoclonal antibodies limited by this antigen is not inventive. However, if the invention is further limited by the hybridoma of monoclonal antibody secreting the antigen, and therefore produces unexpected effects, the invention of the monoclonal antibody is inventive.
The rest of this section has not been modified.
This decision will take effect on January 15, 2021